Abstract
Viral hepatitis B and Viral hepatitis C are one of the leading causes of disease and death in the present-day world. These viruses greatly affect the liver by causing inflammation, cirrhosis, fibrosis, hepatocellular carcinoma (HCC) and even causes the death of affected individuals. It requires highly cautious treatment to resolve complications associated with it, including abnormal levels of serological molecules of specific antigens, antibodies and liver enzymes. The treatment involves the use of antiviral agents that fight against viral antigens, reverses the progression of the disease and also prevents morbidity. Further reading takes towards the development of safe and effective antiviral agents and other regimens for lessening complications and ensuring patient compliance. Moreover, vaccines are also available that fight against the virus and act to prevent the emergence of viral hepatitis. Future developments are today in clinical trials to produce safer and efficacious agents to combat and prevent this disease.
Key Words:
Hepatitis, Disease, Hepatitis B, Hepatitis C, Infection, Infection Control, Treatment
Introduction
Viral hepatitis has been a global health issue over the past centuries. HBV and HCV are defamed for their ruthless, transient and chronic infections on the liver that may cause liver inflammation, cirrhosis, fibrosis, HCC and ultimately can cause liver failure. (Schuppan and Afdhal, 2008) Over periods of 20 to 25 years, compensated cirrhosis can advance to decompensated cirrhosis in 5 to 20% of patients, ultimately ending in hepatic failure and death. (Strader et al.,1996) (Seeff et al.,2002) (Fattovich et al.,1997). Hepatocellular carcinoma is the major cause of death in patients infected with HBV and HCV. (El-Serag, 2012) This deadly virus has infected greater than 300 million people in the world (approx. 5% of the world's population) and brings one million deaths each year. (Robert et al.,1996).
Hepatitis can be both acute or chronic infections. An acute infection can become chronic if the body cannot clear the virus. The majority of the acute cases turns into chronic ones over time. And the majority of the cases are asymptomatic during childhood and during young adulthood. (Bortolotti et al., 1990) (Bortolotti et al., 1998). Moreover, cirrhosis and HCC are rare during childhood but are more frequent in adulthood. Besides HBV (with genotypes ranging from A-H), HCV consists of a group of viruses that are classified into 6 genotypes which are further divided into various subtypes.
The route of transmission the virus adopts is blood and semen. It can transmit among people by coming in contact with bodily fluids. HBV is identified by different serological markers, which are HBV surface antigen (HBsAg) and HBV antibodies (anti-HBs), HBV e antigen (HBeAg) and HBV e antibodies (anti HBe), HBV core antibodies (anti-HBc), IgM and IgG antibodies and alanine transaminase (ALT). (Mast et al., 2005) Likewise, HCV is identified with its respective screening tests for HCV RNA serum levels.
The technique to win the battle against this virus is to suppress HBV / HCV replication such that its progression would be reduced. Such agents have been prepared, which act better to fight this virus with minimum resistance acquired against it. Different strategies have been in use against it along with some new coming ones. People with high levels of hepatitis virus replication and with ongoing / progressed liver disease should be treated with drug regimens as they are prone to death by this infectious agent and are having symptoms. However, keep on checking the other patients to begin the treatment as indications appear.
Goals for HBV and HCV Treatment
The goal of the treatment is to sufficiently stop the progression of viral replication, lessen the inflammation and prevent the development of cirrhosis and HCC. In the case of hepatitis B, this goal is achieved by suppressing the HBV DNA levels and by analysing HBsAg loss, reversion of ALT levels to normal ones, improvement in liver histology and HBeAg seroconversion to anti-HBe. Moreover, IgM, IgG antibodies and anti-HBc are also analyzed. It is obligatory to monitor the treatment response keenly. This is efficacious in detecting virological breakthroughs. Patients adherence to medication should also be closely monitored. In this case the patient fails to succeed with a particular therapy, relocate the cause by studying his history, the characteristics of patients and the disease before the therapy was initiated. Whereas in the case of HCV infection, the most important goal of treatment is to attain sustained virological response (SVR) that is defined as the absence of HCV RNA in the sample serum, that is checked after at least 6 months of stoppage of the therapy. Patients who acquire an SVR at an earlier stage attain a decrease in the HCV RNA levels at 12 weeks into the therapy, which is called early virologic response (EVR). The sustained absence of the detectable virus at the termination of treatment is known as "end of treatment response" (ETR).
Managenent of HBV Infections
The most appropriate way to control the death associated with HBV is through the implementation of antiviral therapy. At present, about six treatment are accepted and are used for hepatitis B, containing interferon (IFN) (having two dosage forms, that are IFN and pegylated interferon [PEG-IFN]), and five FDA-approved nucleoside & nucleotide analogues (NA) are now being used in treatments and these includes (lamivudine, adefovir dipivoxil, entecavir, telbivudine and tenofovir disoproxil fumarate). The expert technique to win against this deadly virus is to initiate the treatment with the agents which possess resistance to a great deal.
Interferon
In 1991, mainstream Interferon alpha-2a (IFN ?-2a) had been launched and approved for the treatment of CHB and was widely adopted, although it has mild efficacy. (Dianzani, 1993) In 2005, IFN had been substituted by Peg IFN? 2a, and it proved to have improved pharmacokinetic parameters and efficacy and less associated treatment complications. Peg IFN therapy continued for a duration of 12 months, causes more than 20% HBeAg seroconversion and more than 20% loss of DNA levels in case of HBV. If this therapy is stopped, after 6 months, the occurrence of HbeAd seroconversion rises to greater than 30%. Six months after discontinuation of therapy, the incidence of HBeAg seroconversion increased to 32%. Almost 60% of the patients get rid of the HBsAg even after quitting the IFN therapy.
This showed the greatest sustained response rate (in the clinical studies) after 1 year of therapy. (Zoulim and Perrillo, 2008) Achieving an early virological response is linked with the development of long term reoccurrence after discontinuation of treatment. (Lau et al., 1997) Despite this, treatment with Peg IFN is less than 10% of all CHB prescription in the U.S. (Zoulim and Perrillo, 2008) This low incidence has been attributed to the significant side effect profile of the drug and the need for injection that is usually to be administered a week thrice.
LAMIVUDINE (LAM)
Lamivudine is a nucleoside analogue that belongs to a class of reverse transcriptase inhibitors. The thing that makes it very attractive in the treatment of CHB is its safety; also, it is the only drug that is available orally in treating CHB. In people with compensated chronic HBeAG positive, it increases the clearance rate of HBV antigen and seroconversion. (Liaw et al., 2000) This therapy is also effective for HB eAg negative patients. (Lau, 2005). Treatment with lamivudine for approximately 5 years causes the development of resistance. (Hann et al., 2008) Different methodologies are used to study the resistance rate of lamivudine, resistance rate, when determined by purely genotypic method with in one year, was found to be near to 32 percent, whereas resistance rate, when determined by virological method, reported the resistance rate of approximately six to fifteen percent so this technique provides a more authentic estimate of resistance. (Chae et al., 2008)
Adefovir Dipivoxil
In 2002, adefovir dipivoxil (ADV) had been used as a nucleotide analogue for the management of CHB, and its emergence has provided a new look at an efficacious treatment. ADV has a definite structure that is responsible for prevention against the emergence of viral resistance. After one year of therapy on ADV, HBeAg seroconversion has been seen in 12% of the patients. (Marcellin et al., 2003) Once it happens, it sustains in 91% of the patients. (Hadziyannis et al., 2003) Yet, the persistence of the viral load even after 48 weeks of treatment is associated with the development of resistance. (Locarnini et al., 2005) But still, resistance occurrence is very much low with ADV as compared to entecavir. (Hadziyannis et al., 2006) (Jonas et al., 2008). In addition, ADV has also shown to be highly effective against LAM resistant HBV infection. (Peters et al., 2004) (Perrillo et al., 2004)
Entecavir
An antiviral drug is belonging to a class of nucleoside reverse transcriptase inhibitor. It is highly effective in reducing the level of HBV DNA in HBeAG positive as well as HBeAG negative patients. (Chang et al., 2006)(Lai et al., 2006)(Seto et al., 2010). This low level of HBV DNA remains after approximately five years of continuous entecavir therapy. (Chang et al., 2010) It is used as a first-line drug in treating hepatitis B cases because it is a higly selective drug and inhibit all the three steps that are involved in the replication of the virus and also because of its low chance for resistance. (Tenney et al., 2009) However, entecavir is not used in patients who are resistant to lamivudine as both of them are nucleoside analogues, and three sites (required for mutations) in entecavir are identical to those that are present in lamivudine (Suzuki et al., 2009)(Karino et al., 2010). Studies show that more than fifty percent of patients who are lamivudine-resistant also develop resistance to entecavir after five years on therapy (Tenney et al., 2009); because of this reason, patients who are resistant to lamivudine are preferably treated with adefovir or tenofovir.
Telbivudine
In 2006, telbivudine, which belongs to the L-nucleoside subgroup, was approved for the treatment of CHB. It is more therapeutically effective than lamivudine for lessening the HBV DNA levels after one year of therapy. (Lai et al., 2007) Study data shows that the chance for developing resistance during treatment with telbivudine is less in patients compared to those patients who take lamivudine, although both of these drugs have the same genetic sites for mutations to cause resistance. Moreover, on the other side, telbivudine has more chance of developing resistance as compared to entecavir and adefovir dipivoxil. (Liaw et al., 2009)
For the selection of patients for keeping the therapy to be continued with telbivudine and lamivudine, attainment of lessened/reduced HBV DNA levels at an early stage is an important criterion. If HBV DNA levels are still identifiable after 24 weeks with treatment with lamivudine, then it is considered to be lamivudine resistance that means that lamivudine is not sensitive in such a case, and it makes it necessary to go for the other drugs (Yuen et al., 2001) Moreover, in addition to this, ALT levels should also be measured, so that an assessment can be done to check that whether treatment with telbivudine and lamivudine is sensitive enough for the HBV infection or not, thus helping in the proper selection of the patients.
Tenofovir Disoproxil Fumarate
An FDA-approved drug for adults as well as for children of 12 years and older causes a very strong suppression of HBV DNA levels, thus having similar action as that of telbivudine and entecavir. Also, it is efficacious against HBV infection with lamivudine resistance and has more therapeutic efficacy than adefovir dipivoxil. Likewise, entecavir is very less prone to develop resistance, as the data does not show any of such cases of resistance even after 4 years of its present-day therapeutic use. Thus, it is considered now to be the FIRST LINE AGENT for the untreated patients and also for the patients who are resistant to lamivudine.
Agent Under Trials
LB80380, a nucleotide that belongs to the group the same as that of tenofovir and adefovir. Clinical trials have shown that this nucleotide has a potent effect, as regards its suppressive action on the virus in both untreated patients and patients having resistance against lamivudine. (Yuen et al., 2006) Its action against acyclic phosphate (tenofovir) resistance is expected to be low. Phase II trials are currently underway in this regard in untreated patients.
Current Regimen to Treat Transmission of HBV from Mother to Child (MTCT)
The deadly virus transmits from the mother to the child during birth if the mother is positive for HBsAg. It has a high risk to occur at the time of delivery or close to it. However, this can be protected by the administration of Hepatitis B immunoglobulin and hepatitis-B vaccine to infants within 12 hours after birth. However, the follow up includes two more doses of vaccines to be administered within 6–12 months of age. There is a possibility to reduce 95% of its chances for the occurrence; however, it is still transmitted from 8-30% of mothers. But before this, make sure that mothers are the best candidates to adapt to this intervention. The risk of MTCT is assessed by reviewing the risk factors (including the level of HBV DNA > 200,000 IU, which constitutes to be 106 copies/ml) and by viewing the literature under the commandment of qualified physicians. They do this by assessment using an algorithm so that effective therapies can be viewed.
We can also check the presence of HBeAg to get an idea about the working of this methodology to prevent HBV infections in newborns. (Xu et al., 2002) Antiviral therapy should be started after the first trimester, i.e., during 4-9 months of pregnancy, so that best results can be obtained in mothers who are prone to the greater disaster of viremia. Elected cesarean section is also effective. However, if the vaccine is administered to the mother long before childbirth, it would be useless against HBV transmission. Breast-feeding by mothers had been avoided to test whether it would be effective against virus transmission, but it had been proven to be useless, provided that immunoprophylaxis is provided to the child. (Euler et al., 2003)(Hill et al., 2002). Moreover, mothers who are HBsAg positive are recommended to be checked for the possible viral transmission to infants so that the child is given timely, effective immunoprophylaxis.
Ganciclovir Treatment of HBV Infection in Liver Transplant Recipients
HBV infections might occur after a liver transplant. In such infections, ganciclovir is efficacious. Ganciclovir is administered intravenously for 3-10 months. The dose ranges from 5mg-10 mg/kg per day. The treatment is gently continued, where drug therapy progresses safely. Ganciclovir shows remarkable efficacy in reducing serum HBV levels from 40% to 90%. It also reduces the mean serum ALT levels by 83%, thus normalizing them. Overall, the therapy reduces the HBV antigens and their replication after a liver transplant. Different vaccines are now being produced after knowing the transcription and replication of the virus and also by keeping in view the structures of integrated HBV sequences in HCC.
Managenent of HCV Infections
Previous Treatments of HCV Infection
Early treatment options included interferon and ribavirin for the attainment of an SVR but had been linked to noteworthy side effects and poor effectiveness. These options had been adopted for HCV-infected children as well. In the 1990s, the first therapeutic regimen for HCV infection had been launched and included non-pegylated interferon alpha 2a or alpha 2b monotherapy. It had the duration of treatment lasting from 24 to 48 weeks, with the dosing of three injections per week (depending on the type of HCV genotype). But by the time of the end of treatment with this regimen, SVR had not been achieved due to a lot of side effects and for being clumsy to the patients. After this, the FDA approved two genetically engineered alpha-interferon as starting treatment for HCV infection. In 1991, a recommendation was adopted that 3 million units for the interferon alfa 2b can be administered with weekly dosing of three times and for a duration of 6 months. Again in 1996, the use of 3 million units had been approved, with subcutaneous administration, with weekly dosing for 3 times, and for a duration of 12 months. Yet, in clinical trials, the highest SVR had been attained with the use of Peg-INF and ribavirin. Peg-INF proved to be longer acting that made the short duration of therapy possible, from conventional 3 weeks subcutaneous injection to once weekly administration but had the same side effects as that of INF. (Manns et al., 20
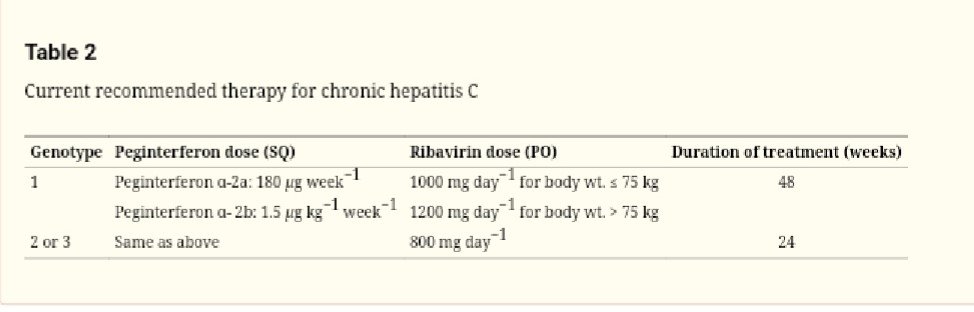
Recommended Doses of Peg-INF and Ribavirin for HCV with Stipulated Treatment Duration
Some other drugs that had been investigated for the treatment of HCV infection consisted of lymphoblastoid interferon-alpha (interferon-alpha n1), interferon derived from leukocyte, consensus interferon, and some other beta interferons.
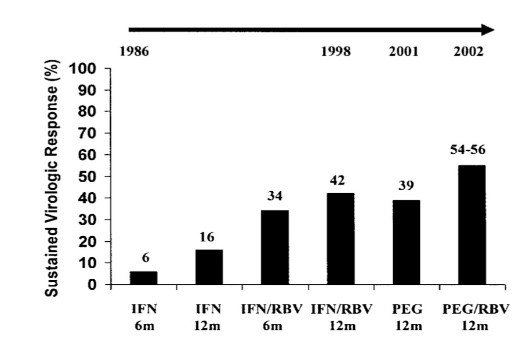
Landmarks in the Therapy of CHB.
(m: months, IFN: Interferon, RBV: Ribavirin, PEG: Pegylated Interferon)
Current Treatment Options to HCV Infection
A remarkable advancement in the treatment of HCV infection came when Peg IFN was introduced after chemical modifications in interferon-alpha molecule, which proved to have better pharmacokinetic parameters, including improved half-life as compared to previous interferon formulations. As far as a treatment for HCV infection is concerned, thegenotype of HCV is the most crucial point in deciding length for the duration of treatment and therapeutic outcome. According to practical treatment procedures, for genotype 1, 48 weeks are recommended to be continued with the treatment regimen (SVR around 40%). And same is the case for genotypes 4, 5 and 6. There is no separate established treatment protocol for these three genotypes, and so they are treated with the same regimen as that for genotype 1. Whereas, for genotypes 2 and 3, 24 weeks of treatment with ribavirin and Peg IFN are recommended (SVR around 75%). Concluding, treatment, including Peg IFN, proved to have far better results as compared to monotherapy. The present-day recommended schedule for HCV is as follows
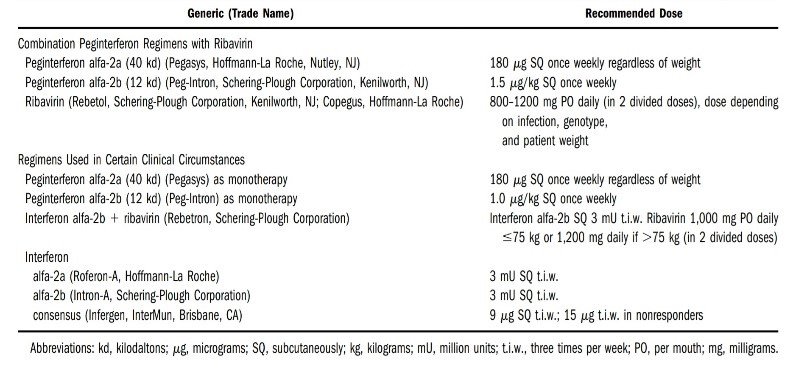
Recommended therapy for CHB with Peg-INF and Ribavirin with Dosing Schedules
After 2010, direct-acting antiviral agents (DAAs) had been launched, and to date, these agents have proved to have better efficacy, provided much tolerance to patients during treatment and reduced mortality to a great extent with cure rates of greater Figure 3: Recommended therapy for CHB with Peg-INF and ribavirin with dosing schedules.
than 95%. These agents have also exposed the various new alternative ways to treat patients with HCV infection corresponding to different genotypes and having other coexisting morbidities as well. Moreover, these have also made possible the duration of treatment to be shorter, so increasing patient compliance. This has made the use of IFN obsolete.
Telaprevir or Boceprevir Combination with Peg-Inf And Ribavirin
In 2011, first DAAs [telapravir (Jacobson et al., 2011) and boceprevir (Poordad et al., 2011), both being protease inhibitors causing inhibition of HCV replication], had been used with Peg INF alpha plus weight-based ribavirin and are now being included in the established treatment protocol for HCV genotype 1 infection. Seven evidence-based randomized clinical trials in patients having HCV genotype 1 infection showed the superiority of the above regimen (SVR 61-75%) against the sole combination of Peg-INF and ribavirin (SVR 38-49%). (Sherman et al., 2011) This new combination also showed improvements in the SVR rates of previous partial responders and also in patients who relapsed, with SVR rates reaching 69-83%, as compared to the SVR rates of 20-29% in the same patients with the use of Peg-INF and ribavirin. (Muir et al., 2011) On the other side, there are serious adverse reactions that are associated, which put emphasis on the fact that improvement is needed. So in this regard, another DAA, simeprevir, was launched and was included in the regimen with ribavirin and Peg INF alpha for HCV genotype 1 and proved to have less adverse effects, yet with similar SVR rates as compared to the previous combination. (Fried et al., 2013) In the same time period, sofosbuvir was launched and showed to be efficacious against all the six genotypes of HCV. Upon performing clinical trials, it was found that DAAs are also solely efficacious against genotype 1 with SVR of greater than 90% and are also known to have fewer side effects. Around 2014, the FDA approved regimens for sofosbuvir/ledipasvisr and sofosbuvir/simepravir. Currently, there are various DAAs available that are least prone to develop resistance, which are grouped into;
? Protease NS3 inhibitors including peritaprevir, glecapravir, voxilaprevir, simeprevir and grazoprevir.
? NS5A serine protease inhibitors including ombitasvir, velpatasvir, daclatasvir, probrentasvir, ledipasvir and elbasvir.
? NS5B RNA dependant RNA nucleoside polymerase, including sofosbuvir.
? Non-nucleoside polymerase inhibitors, including dasabuvir.
WHO recommends the inclusion of DAAs in the treatment protocols for treating chronic HCV infection in patients of 18 years or more. Whereas, for patients with chronic HCV infection, of age ranging from 12-17, WHO has the following recommendations;
? Sofosbuvir/ledipasvir combination for 12 weeks (genotypes-1,4,5 and 6).
? Sofosbuvir/ribavirin combination for 12 weeks (genotype 2).
? Sofosbuvir/ribavirin combination for 24 weeks (genotype 3).
Sofosbuvir Combinations
The novel anti-HCV drug, sofosbuvir, when used with daclatasvir, ledipasivir, ribavirin and interferon, proved to be greatly efficacious in treating interferon resistant repititive HCV infection and preventing interferon related adverse drug reactions (ADRs).
Sofosbuvir had been investigated as a substitute for interferon, and when used with ribavirin, it proved to be a highly effective substitute for reducing the viral RNA levels beyond being quantified (Gane et al., 2013). An important clinical trial study included one untreated and the other previously treated group of patients with HCV infection of genotypes 2 and 3 and had been treated with the regimen of sofosbuvir and ribavarin. (Osinusi et al, 2013) The included patients showed undetectable RNA levels (97% clinical outcome compared with 78% response with Peg INF). (Jacobson et al, 2013)
Sofosbuvir with Ledipasivir
This combination exhibit high SVR (approximately 90%) in patients with HCV genotype 1 infection, as both sofosbuvir and ledipasivir are active against this genotype in HCV patients, also including solid organ transplant recipients and patients with mild to moderate renal impairment. (Balistreri et al., 2017) This combination is found to be superior to the combination of ledipasivir, sofosbuvir and ribavirin as regards the adverse effects. But is active against genotype 1a infection in only treatment-naive patients (TNPs) and not in treatment-experienced patients (TEPs). (EASL, 2017) Though this combination is effective for genotype-1b in both treatment-naive (SVR rate 96-97%) and treatment experienced patients (SVR rate 87%). And these clinical results comply with the real world studies. Active against genotype 4 (without having liver cirrhosis or having compensated cirrhosis)(SVR rate 95%) (Kohli et al, 2015), genotype 5 and genotype 6 (for TNPs not having cirrhosis or with compensated cirrhosis) having SVR rate of 95% (Abergel et al., 2016) and 96% respectively.
Sofosbuvir with Daclatasvir
This combination has broad HCV genotypic coverage and is an active drug combination against CHB. In the clinical investigations, it exhibited a high SVR as compared to the drug treatments with telaprevir and boceprevir. This regimen also proved to be highly effective in patients who had undergone a liver transplant in comparison to the conventional combination of Peg-INF and ribavirin, because it possess less side effects and more antiviral action. And this makes this combination, the most efficacious and safe for patients having liver transplant with repititive HCV infection.
Sofosbuvir with Velpatasvir
Active against HCV genotype 1 in TNPs and in TEPs, whether having cirrhosis or not. Had SVR rate of 98% in clinical trials in genotype 1 infected patients. (Feld et al., 2015) Also active against genotype 2 (SVR rate 99%)(Foster et al., 2015), genotype 3 (without having liver cirrhosis)(SVR rate 98%), genotype 4 (without having cirrhosis or having compensated cirrhosis)(SVR rate 100%) (Feld et al., 2015), genotype 5 (without having cirrhosis or with compensated cirrhosis)(SVR rate 97%)(Feld et al., 2015) and against genotype 6 (for TEPs and TNPs, without having cirrhosis or having compensated cirrhosis)(SVR rate 97%)(Lim et al., 2017) These results are being confirmed in the real world studies as well.
Glecaprevir with Pibrentasvir
Effective in TNPs and TEPs without having liver
cirrhosis or having compensated cirrhosis against genotype 1a (SVR rate 98%), genotype 1b (SVR rate 100%), genotype 2 (SVR rate 98%). Also active against genotype 3 (SVR rate 95% in TNPs without liver cirrhosis but having fibrosis, 98% in TNPs having cirrhosis, 100% in patients who are coinfected with HIV but are not having cirrhosis, 99% in TNPs and 94% in TEPs having compensated cirrhosis, 91% in TEPs, not having cirrhosis. (Wyles et al., 2018) This combination is also active against genotype 4 (SVR rate 93% in patients not having cirrhosis and SVR 99% in patients having cirrhosis) and genotype-5 (in TNPs and TEPs with SVR rate 100% in patients not having cirrhosis). (Asselah et al., 2018) Moreover, it also posses activity against genotype 6 (in TNPs and TEPs whether having cirrhosis not) with an SVR rate of 90% in cases without cirrhosis (Kwo et al., 2017) and SVR rate of 100% in cases with cirrhosis. (Asselah et al., 2018)
Grazoprevir with Elbasvir
Active against genotype 1a (without liver cirrhosis or having compensated cirrhosis) in treatment-naive, having SVR rate 97% (Rockstroh et al., 2015) and in TEPs with SVR rate 92% (Kwo et al., 2017), genotype-1b (without cirrhosis or having compensated cirrhosis) in TNPs with SVR rate 99% (Zeuzem et al., 2015) and in TEPs with SVR rate 100% (Kwo et al., 2017), genotype 4 (without cirrhosis or having compensated cirrhosis) in
Drawback of Daas
Includes much medicine-related cost and not having any coverage for insurance regarding some antivirals. In addition to this, re-infection is an unavoidable risk.
Assessment of Treatment Responses in Hbv and Hcv Infections
Treatment response for hepatitis B is assessed by a decrease in the levels of HBV DNA, elimination of HBeAg, restoration of ALT levels, elimination of HBsAg and also reduction in the liver inflammation in liver biopsy. It is recommended to perform an examination of serum ALT and levels of HBV DNA for one complete year, with intervals of 3 months of gap. After one year, one should be examined with a time-lapse of 6 months to 12 months so that repetitive exacerbations of HBV can be detected. (Keeffe et al., 2008) This routine is likewise valuable to separate constant dynamic HBeAg negative patients from dormant transporters. Treatment should be started paying little heed to the degree of viremia if dynamic irritation is additionally distinguished on liver biopsy. (Wiegand et al., 2010) If a patient is HBeAg negative, treatment for CHB is to be continued to such an extent that the HBsAg becomes unidentifiable. However, keen observation and examination are important to achieve maximum therapeutic outcomes. Treatment considerations are also based on the type of HBV genotype, which ranges from genotype A to genotype H. (Palumbo et al., 2007). Children who are infected with HBV genotype B and C have a greater prevalence for the presence of HBeAg and also have high levels of HBV DNA when compared with other HBV genotypes. Genotype C is being the most problem causing genotype, having the greatest risk for HCC. (Chan et al., 2004) Moreover, in the case of CHB, not all children would benefit from antiviral therapy due to the development of resistance to such antivirals and due to severe side effects. So, treatment should be considered for children in keeping view of the active CHB, presence of fibrosis or if liver biopsy examination shows inflammation ranging from moderate to severe level. (Jonas et al., 2010)
Whereas considerations for HCV treatment include disease severity, i.e., acute versus chronic, laboratory reports, genotype, co-infection and comorbidities. These are important factors that can affect treatment outcomes and period of antiviral treatment and also holds for as a stopping rule for treating patients.

Flowchart representing sequential steps for CHB (A) for management of patients with genotype 1 (B) for management of patients with genotype 2 and genotype 3.
(SVR: sustained virological response, ETR: end of treatment response).
Hepatitis B and C Vaccines Hepatitis B Vaccines
For taken as alternative strategies, various vaccines have been developed for CHB and have shown efficacious treatment outcomes as regards clinical trials are concerned.
Vaccines Nased on Proteins
Those Containing Particles of Hepatitis B Antigen
In 1982, the development and introduction of a prophylactic vaccine for HbsAg-Based efficiently decreased the HBV infection. It is basically an immune therapy for CHB infection and proved its safety and efficacy in clinical trials.
Yeast Derived Immunogenic Complex (Yic)
The phase 1 studies and phase 2 studies showed that YIC proved to be safe, efficacious and potent in the treatment and management of patients with CHB infection.
Hbsag Combines Antiviral Drugs
In clinical trials, the specific immune responses had been induced by HBsAg based vaccines; also, it reduced the load of the virus in the clinical trials, so there would be known significant effects for HBV, DNA and HBeAg seroconversion clearance. The antiviral drugs and therapeutic vaccine combinations exhibit therapeutic effects which remained sustained in animals. In 5 trials, the LAM was combined with Pre S1/Pre S2/S vaccines to check whether it is effective in suppressing the virus in patients with HBV infection or not. In these trials, there had been greater effective viral suppression as well as anti-HBs response in the treated group, but this effect vanished after approximately 1.5 years.
Anti HBV Vaccines Containing HBSAG and HBCAG
HBcAg inhibits viral infection by the activation of the body's natural defence mechanisms. It includes specific T-cells and anti-HBs production. These vaccines work by activating antigen-presenting cells, i.e. B lymphocytes. Also, it possesses synergistic efficacy overproduction of antibody and other responses of the cell when it is given in combination with HBsAg.
Other Protine Based Vaccines
GS-4774 is a genetically engineered product based on yeast, which is designed for expressing HBV antigens. This product enhances the host defensive response in patients with CHBinfection. Clinical studies have shown that it is safe and is tolerated well in patients with HBV infection. However, it does not significantly reduce the levels of HBsAg in the serum of patients, so it does not have significant therapeutic and clinical outcomes.
DNA based Vaccines
The DNA based vaccine for HBV is being investigated by researchers around the globe because of the advantage of induction of humoral immune response and cellular immune response both at the same time. pCMV S2 S is a DNA based vaccine that is considered to be safe and is able to activate the response of T lymphocytes in certain carriers, but it has weak and transitory action.
Vaccines Containing Live Vectors
Such vaccines stimulate the production of specific antigens in host cells and activate host defensive immune responses. Para pox virus, herpes virus and adenoviruses are frequently used in the production of such live vector vaccines that are effective against HBV. TG1050 is a vaccine based on adenovirus, which modifies HBV polymerase. Preclinical studies have shown that TG1050 had a potent effect on HBV infection.
Hepatitis C Vaccines
The development of a safe and effective HCV vaccine is thou tough task, and many impediments have to be recognized. Developmental barriers include lack of persistent and susceptible small animals, a very high degree of HCV failure & genomic diversity of HCV in tissue cultures. Nevertheless, it might be still very difficult to produce a vaccine that uniformly gives sterilizing immunity. Vaccines observed so far includes the following;
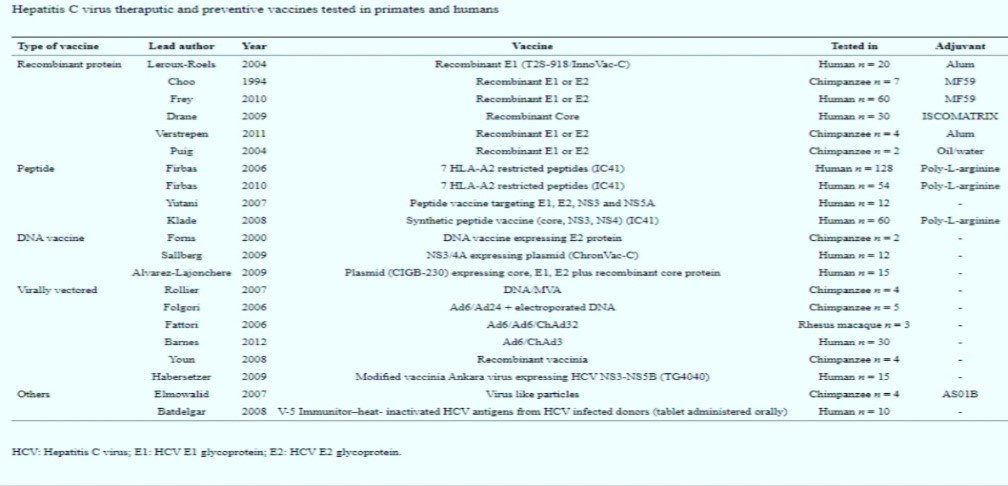
HCV Vaccines Tested in Primates and in Humans
Challenges to Development of Hepatitis C Vaccine Hcv Genetic Variability
Studies show that HCV mutates nearly 1 nucleotide per-cycle of replication. The leads to very high genetic variability, resulting in different but closely related variants of HCV. It is estimated that HCV is a 10 times greater variable than HIV, and this causes significant challenges for the development of a successful vaccine.
HCV Persistence
Mitochondria are attacked by HCV in liver cells and causes induction of mitochondrial dysfunction and oxidative stress that results in the induction of autophagy mitophagy of the cell. The proteins which cause autophagy acts as a proviral factor that starts the translation of HCV RNA (currently immune surveillance), which gives way for infection in liver failure.
Lack of an Appropriate HCV Model
This is due to the concern of ethical considerations and due to variability of results in the same animal models that make the interpretation of the results quite difficult. Another factor is the associated cost in the maintenance of animal models and to carry out further studies on them.
Moreover, the pharmaceutical companies are investing more in drug development; vaccine development requires investment from the government, charitable foundations and from sources beyond government.
Future Directions for Vaccines
There are significant deficits in the tools for the development of effective and safe HCV vaccines. Elucidating the mechanism by which antigen-specific immune cells cause long term protection is an important goal. Vaccine strategy should be to overcome the enormous diversities of HCV infections. For this, it must generate a broad and effective immune response, which would be capable of responding to abundant variation. Selecting antigens that would maximize the inductions of antibody response and T cells remains an active area of research. Moreover, the successful control of HCV infection could be possible by the use of a combination of drugs, large scale screening to identify individuals who are infected, harm reduction strategies and preventive measures for those who are not infected but at risk. The development of a prophylactic and safe HCV vaccine is still a worthy challenge.
Conclusion
Development in the way of producing antiviral agents against HBV and HCV has been a long process, and a huge number of clinical trials are being performed in order to develop safe and effective treatments for the infected patients. Various antivirals are developed for these sort of infections. Although treatment with NAs is currently in clinical practice, more effective therapies are urgently needed for more safe and efficacious treatment. Postmarketing surveillance of NA reported severe side effects, including mitochondrial dysfunction, myopathy, neuropathy, nephrotoxicity and pancreatitis. So, careful monitoring is required during the whole duration of treatment so as to prevent and avoid the severity of morbidity associated with HBV and HCV and to reverse the progression of such diseases. And before treatment, the patients should be sufficiently assessed for the type of therapy that is the most suitable, safe and effective for them. The same is the case of DAAs that have been developed for the HCV infection, and such developments have led to the finding and production of a lot of antiviral drugs with remarkable efficacy. Clinical trials with highly effective DAAs are underway in the children. The coming times look forward to the development of effective vaccines by overcoming the obstacles posed in their way.
References
- Abergel A, Asselah T, Metivier S, Kersey K, Jiang D, Mo H, et al. (2016). Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis;16:459-464.
- Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, et al. efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol 2018;16:417-426.
- Balistreri, W.F., Murray, K. F., Rosenthal, P., Bansal, S., Lin, C.H., & Kersey, K. et al. (2017). The safety and effectiveness of ledipasvir- sofosbuvir in adolescents 12-17 years old with hepatitis C virus genotype 1 infection. Hepatology; 66:371-378
- Bortolotti, F., Cadrobbi, P., & Crivellaro, C. et al. (1990). Long-term outcome of chronic type B hepatitis in patients who acquire hepatitis B virus infection in childhood. Gastroenterology. 99:805-10.
- Bortolotti, F., Jara, P., & Crivellaro, C et al. (1998). Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol.;29:184-90.
- Buster, E. H., Hansen, B. E., Lau, G. K., Piratvisuth, T., Zeuzem, S., Steyerberg, E. W., & Janssen, H. L. (2009). Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology.137:2002-2009.
- Chae HB, Hann HW. Baseline HBV DNA level is the most important factor associated with virologic breakthrough in chronic hepatitis B treated with lamivudine. World J Gastroenterol. 2007;13:4085-4090.
- Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494-8.
- Chang TT, Gish RG, de Man R al. et A comparison of entecavir and lamuvidine for HBe Ag- positive chronic hepatitis B. N. Engl. J . Med 2006; 354: 1001-10.
- Chang, T. T., Lai, C. L., Kew Yoon, S. al et. (2010). Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positivechronic hepatitis B. Hepatolog; 51: 422- 30.
- Dianzani F. Biological basis for the clinical use of interferon. Gut. 1993;34:S74-S76.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.
- Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis B surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics. 2003;111:1192-7.
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol 2017;66:153- 194
- Fattovich G, Giustina G, Degos F, Diodati G, Tremolada F, Nevens F, Almasio P, et al. effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol 1997;27:201-205.
- Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV gGenotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599-2607.
- Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373:2608-2617.
- Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
- Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368(1):34-44.
- Gish RG, Chang TT, Lai CL al et. Loss of HBsAg antigen during treatment with entecavir or lamuvidine in nucleoside-naive HBe Ag- positive patients with chronic hepatitis B. J. Viral. Hepat. 2010; 17: 16-22.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800- 807.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg- negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743- 1751.
- Hann HW, Gregory VL, Dixon JS, Barker KF. A review of the one-year incidence of resistance to lamivudine in the treatment of chronic hepatitis B: Lamivudine resistance. Hepatol Int. 2008;2:440-456.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839-1850.
- Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99:1049.
- Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416
- Jonas MM, Block JM, Haber BA, et al. treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology. 2010;52:2192-205.
- Jonas MM, Kelly D, pollack H, et al. Safety, efficacy, and pharmacokinetics of adefovir dipivoxil in children and adolescents (age 2 to
- Karino Y, Toyota J, Kinda H al et. efficacy and resistance of entecavir following 3 years of treatment of Japanese patients with lamuvidine refractory chronic hepatitis B. Hepatol. Int. 2010; 4: 414-22.
- Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-141; quiz 1286.
- Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of- concept, single-centre, open-label Phase 2a cohort study. Lancet Infect Dis 2015;15:1049-1054.
- Kwo P, Gane EJ, Peng CY, Pearlman B, Vierling JM, Serfaty L, et al. effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment- experienced patients with chronic hepatitis C infection. Gastroenterology 2017;152:164-175, e164.
- Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol 2017;67:263-271.
- Lai CL, Gane E. Liaw VF al et. Telbivudine versus lamuvidine in patients with chronic hepatitis B. N. Engl. J. Med. 2007; 357: 25 76-88.
- Lai CL, Shouval D, Lok AS al et. entecavir versus lamuvidine for patients with HBe Ag- negative chronic hepatitis B. N. Engl. J. Med; 2006; 354: 1011-20. Seto WK, Lai CL. Fung J, Yuen J, Wong DKH, Yuen MF. A three year study on viral suppressions and resistance profile for treatment-naive CHB patients receiving continuous entecavir treatment. Hepatol. Int; 2010; 4: 58.
- Langley DR, Walsh AW, Baldick CJ al et. inhibition of hepatitis B virus polymerase by entecavir. J. virol. 2007; 81: 3992-4001.
- Lau DT, Everhart J, Kleiner DE, Park Y, Vergalla J, Schmid P, Hoofnagle JH. Long-term follow up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology. 1997;113:1660-1667.
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682- 2695.
- Liaw YF, Gane E, Leung N al et.2-year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009 ; 136: 486-95.
- Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180.
- Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531.
- Lim SG, Mohamed R, Le P, Tee HP, McNabb BL, Lu S, et al. safety and efficacy of sofosbuvir/velpatasvir in a genotype 1-6 HCV-infected population from Singapore, Malaysia, Thailand, and Vietnam: results from a Phase 3 clinical trial. Hepatology 2017;66:586A.
- Locarnini S, Qi W, Arterburn S. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB) Hepatology. 2005;42:17A.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662.
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958 -965.
- Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816.
- Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192:1880-1889.
- Muir AJ, Poordad FF, McHutchison JG, et al. Retreatment with telaprevir combination therapy in hepatitis C patients with well characterized prior treatment response. Hepatology. 2011;54(5):1538-1546.
- Osinusi A, Meissner EG, Lee YJ, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial.JAMA. 2013;310(8):804-811.
- Palumbo E. Hepatitis B genotypes and response to antiviral therapy: a review. Am J Ther. 2007;14:306-9.
- Papatheodoridis GV, Manesis E, Hadziyannis SJ. The long-term outcome of interferon-alpha treated and untreated patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2001;34:306-313.
- Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81-90.
- Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine- resistant chronic hepatitis B. Gastroenterology. 2004;126:91-101.
- Poordad F, McCone J Jr, Bacon BR, et al; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195-1206.
- Robert G Gish, JY Lau, Linda Brooks, Jane WS Fang, Stephen L Steady, Joanne C Imperial, Richard Garcia-Kennedy, Carlos O Esquivel, Emmet B Keeffe Hepatology 1996;23 (1), 1-7.
- Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, et al. efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C- EDGE CO-INFECTION): a non- randomised, open-label trial. Lancet HIV 2015;2:e319-e327.
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet.2008;371:838-851.
- Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. HEPATOLOGY 2002;36.
- Sherman KE, Flamm SL, Afdhal NH, et al; ILLUMINATE Study Team. Response- guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365(11):1014-1024.
- Strader DB, Seeff LB. The natural history of chronic hepatitis C infection. Eur J Gastroenterol Hepatol 1996;8:324 -328.
- Suzuki Y, Suzuki F, kawamura Y al et. efficacy of entecavir treatment for lamivudine- resistant hepatitis B over 3 years: histological improvement or entecavir resistance? J. Gastroebterol. Hepatol. 2009; 24: 429-35.
- Tenney DJ, Rose RE, Baldick CJ al et. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rae through 5 years of therapy. Hepatology 2009; 49: 1503-14
- Wiegand J, van Bömmel F, Berg T. Management of chronic hepatitis B: status and challenges beyond treatment guidelines. Semin Liver Dis. 2010;30:361-377.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-618.
- Yuen MF, Kim J, Kim CR al et. A randomized placebo- controlled, dose-filling study of oral LB80380 in HBeAg- positive patients with chronic hepatitis B.Antivir,Ther,2006;11:977-83.
- Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785-791.
- Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015;163:1-13.
- Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48 Suppl 1:S2-19
Cite this article
-
APA : Zahid, M., Hamid, A., & Asad, A. (2017). A Review on the Management of Chronic Hepatitis B and C Infection. Global Immunological & Infectious Diseases Review, II(I), 59-62. https://doi.org/10.31703/giidr.2017(II-I).05
-
CHICAGO : Zahid, Marriam, Ayesha Hamid, and Aleenah Asad. 2017. "A Review on the Management of Chronic Hepatitis B and C Infection." Global Immunological & Infectious Diseases Review, II (I): 59-62 doi: 10.31703/giidr.2017(II-I).05
-
HARVARD : ZAHID, M., HAMID, A. & ASAD, A. 2017. A Review on the Management of Chronic Hepatitis B and C Infection. Global Immunological & Infectious Diseases Review, II, 59-62.
-
MHRA : Zahid, Marriam, Ayesha Hamid, and Aleenah Asad. 2017. "A Review on the Management of Chronic Hepatitis B and C Infection." Global Immunological & Infectious Diseases Review, II: 59-62
-
MLA : Zahid, Marriam, Ayesha Hamid, and Aleenah Asad. "A Review on the Management of Chronic Hepatitis B and C Infection." Global Immunological & Infectious Diseases Review, II.I (2017): 59-62 Print.
-
OXFORD : Zahid, Marriam, Hamid, Ayesha, and Asad, Aleenah (2017), "A Review on the Management of Chronic Hepatitis B and C Infection", Global Immunological & Infectious Diseases Review, II (I), 59-62
-
TURABIAN : Zahid, Marriam, Ayesha Hamid, and Aleenah Asad. "A Review on the Management of Chronic Hepatitis B and C Infection." Global Immunological & Infectious Diseases Review II, no. I (2017): 59-62. https://doi.org/10.31703/giidr.2017(II-I).05
