01 Pages : 1-7
Abstract
Blood collection from experimental animals is a common yet crucial procedure that requires immense care and professional experience to ensure propriety and successful trials in the experimental procedure. This article overviews different methods for blood collection, terminal and non-terminal, in different experimental animals, mainly rat, guinea pig, and rabbit. Different sites for the collection of blood have been discussed, along with the pros and cons of administering via these sites. Lastly, it is discussed that the site of selection depends on the sample volume required and the purpose of the experimental procedure; these factors are also discussed in detail in this review article.
Key Words:
Different blood collection techniques are employed while collecting the blood of experimental animals. These techniques can be broadly classified as terminal and non-terminal; the procedures for collecting blood mainly focuses on rat, guinea pig, rabbit and mice. From the general principles of blood collection to detailed procedures, all the aspects have been covered in this article. The practical application of anti-coagulants has also been discussed with examples.
Introduction
Different blood collection techniques are employed while collecting the blood of experimental animals. These techniques can be broadly classified as terminal and non-terminal; the procedures for collecting blood mainly focuses on rat, guinea pig, rabbit and mice. From the general principles of blood collection to detailed procedures, all the aspects have been covered in this article. The practical application of anti-coagulants has also been discussed with examples.
General Principles of Blood Collection in Animals
• The technique of blood collection needs to be defined in the protocol authorized by the Institute animal ethics committee.
• It needs to be the least painful and stressful. Sample of blood may be collected under or without anaesthesia. (Dang et al., 2008)
• Sufficient training is needed for blood collection using any technique in any species.
• In general, a sample of blood is withdrawn from venous, arterial blood vessels or heart chambers.
• The frequency of collection of blood is vital. Once in two weeks is perfect for nonrodents. Lagomorphs (e.g., hares and rabbit) can be used if the study needs multiple samples of blood.
• All non-terminal collection of blood without replacement of fluids is restricted up to 10% of total circulating blood volume in healthy, normal and adult animals on a single occasion and may be repeated after 3 to 4 weeks. In case repeated blood samples are required at short intervals, a maximum of 0.6 ml/kg/day or 1.0% of an animal’s total volume of blood can be removed every 24 hours.
• The samples can be withdrawn through a temporary cannula if the study involves the repeated collection of the blood sample. This may lessen pain and stress in experimental animals. (Cuper et al., 2011)
General methods for Blood Collection
Mice have around 58.5 ml of blood/kg of body weight on average. A mouse having a weight of 25 g would hence have a (TBV) of approximately 58.5 ml/kg x 0.025 kg = 1.46 ml. (Courtice, 1943)
Decision Tree
The two tables given below are intended to contribute to determining the amount of blood to sample from animal, and depending on that, a volume which is the most appropriate methods to use.
Use of Anti-Coagulants
The type of sample needed must be determined prior to the bleeding procedure. Experimental procedures could need whole blood, plasma, or serum. An anti-coagulant must be added to the sample for whole blood (Birck, Tveden-Nyborg, Lindblad, & Lykkesfeldt, 2014). Plasma, when separated from the red blood cells, can be extracted from an anticoagulated sample. The serum is obtained through the collection of blood without an anti-coagulant. Once a clot has formed, the serum will be the outcome from the centrifugation of the sample (Bardelmeijer et al., 2003). As the sample has clotted, the serum will not comprise fibrinogen or other clotting factors. Both plasma and serum are obtained through the use of a centrifuge, which runs at 2200-2500 RPM for a minimum of 15 minutes.
A suitable anti-coagulant must be used for a sample that must yield whole blood or plasma. anti-coagulants that are commonly used for laboratory animals are:
• Heparin
• sodium citrate
• ethylenediaminetetraacetic acid (EDTA), whose selection is based on research needs.
Withdrawal of Blood sample from Guinea Pig
Requirements
Guinea pig (Cavia porcellus) having weight 700 g, sample collection tubes, gloves, cotton, towel, anaesthesia, 21G, 23G, 25G needle and EDTA.
Procedure
Blood Collection from a Tarsal Vein (Preferred Route)
In this method, there is no need for anaesthesia. Guinea pig is just held with its foot controlled. Before taking the blood sample, a considerate pressure is applied by rubbing over the point of collection so that the vessel becomes dilated. The collection site is trimmed with an electric clipper and cleaned with 70% alcohol and butadiene. 23 gauge needle covered with EDTA is inserted in the tarsal vein of the leg, and 0.2ml blood is withdrawn. To attain hemostasis, the pressure is applied with sterile gauze of around 2 minutes to stop blood flow. Within the period of 24-hour blood samples should not be taken more than six from both hind limbs. (Kalisch, Elbel, Gössl, Czisch, & Auer, 2001)
Withdrawal of Blood Sample from Rat
Requirements
Brown rat (Rattus norvegicus) having the weight of 230g, towel, Gauze size of needles: 21G, 23G, collection tubes
Procedure
For the rat, blood collection is preferred from the tail vein and saphenous vein.
Blood Collection from the Tail Vein
First of all, the tail is cleansed with alcohol (70%). With the help of 21–23-gauge needles, 2ml of blood is withdrawn from the lateral tail vein by restraining the rat. Hemostasis is attained when pressure is applied with sterile gauze to stop blood flow.
Blood collection from the Saphenous Vein
Exactly above the knee joint, considerable pressure is applied to immobilize the hind leg, and the rat becomes restrained. The collection area is trimmed with an electric clipper and cleansed with three alternating scrubs of 70% alcohol. 23-gauge needle is inserted into the lateral saphenous vein, and 0.2ml blood is withdrawn. Hemostasis is attained when pressure is applied with sterile gauze to stop blood flow. (Kowarski, Giancatarino, Kreamer, Brecht, & Kowarski, 1976)
Blood Collection from Retro-Orbital Plexus
Sample taken from the retro-orbital plexus is only one. With recovery, sample volume must be 0.2ml, and without recovery, it must be up to 0.5ml (Matta & Lam, 1997). The equipment used for collection is the Pasteur pipette. To avoid any adverse effect, careful monitoring is required. Adverse effects like Retro-orbital haemorrhage leads to hematoma and excessive pressure on the eye, corneal ulceration, pannus formation, keratitis, rupture of globe and micro-ophthalmia due to proptosis of the globe, damage to the optic nerve and other intra-orbital structures, fracture of fragile bones of orbit and neural damage by micro-pipette, penetration of eye globe itself with loss of vitreous humor. (Parasuraman, Raveendran, Kesavan, & pharmacotherapeutics, 2010)
Blood Collection via Cardiac Puncture
As it is a terminal technique, therefore, only one sample can be taken from a single animal. A sample of up to 15 ml can be withdrawn using a 19 to 21G needle. Usually, one person is enough to perform blood sampling. Slowly withdraw blood to avoid collapsing the heart of an animal.
Blood Collection via the Jugular Vein
Not more than 8 blood samples are recommended to be taken in 24 hours. A sample volume of 0.1 – 2ml can be taken with a 23G needle. Two people are required to perform sampling, one to take a sample and the second to restrain the rat. Talking about adverse effects, Hemorrhage, bruising, and infection can be observed. Rats are detained in an unnatural position, so it can cause stress. To perform this experiment, a high degree of competence is required.
Withdrawal of Blood Sample from Mice
Requirements

Blood sample collection tubes, animal, cotton, Intravenous cannula, towel, 19 to 25G needle, surgical blade, anti-coagulant agent, e.g. heparin, hair removal cream or shaving blade to shave hair when required, disposable bags and thoracotomy tubes with an internal diameter of 0.1 to 0.3 mm.
Blood Vessel Cannulation
This method is usually done in the Jugular vein, dorsal aorta, femoral artery, vena cava, femoral vein and carotid artery. In a 24- hour period, 6 samples can be taken, depending on sample volume. A sample of 0.01 to 0.02 ml can be taken. Usually, one person is enough to withdraw a blood sample. However, more personals are required in case of surgery, post-operative care and post-operative animal observation on a daily basis. As far as adverse effects and incidents are concerned, Hemorrhage blocked cannula, infection, poor recovery, swelling and skin sore can be observed. The weight of mice should be resumed to their pre-operative weight before blood sampling. House your animal singly in the spacious container. Don’t forget to flush the cannula after the
Withdrawal of blood.
Tail Snip Method
This technique should be performed under terminal anaesthesia and should be avoided as far as possible because it causes permanent damage to the tail of the animal. Furthermore, this method is recommended only in the case of blood collection from mice. Use local anaesthesia on the mice’s tail and make a cut at 1mm from the tail’s tip utilizing a scalpel blade. Dabbing an animal’s tail can help in stopping bleeding.
Tail Vein Blood Sample Collection
This is the most preferred route for blood sample collection technique. Considering sample volume, one or two samples can be taken in a 24-hour period. Restrain your animal comfortably, maintaining a temperature of 24 to 27 degrees centigrade. To avoid leukocytosis, don’t rub the tail from base to tip. Try dipping the tail in warm water if the vein is not visible. A sample volume of 50 microliters to 0.2 millilitres can be withdrawn using a 25G needle. One person is enough to perform blood sampling if a restraint tube is used. Infection and Hemorrhage can be observed. One can warm the mice to achieve blood vessel dilation, avoiding dehydration and hyperthermia.
Saphenous Vein Blood Sampling
In a 24-hour period, a maximum of four blood samples can be withdrawn. In the case of the sample volume of 0.15ml, it can be repeated at an interval of 2 weeks without disturbance in haematological status. A lance of 27G or 25G is used to perform the blood sampling. Not more than three attempts should be made. One person is sufficient to perform the blood sampling technique. Haemorrhage, bruising, temporary favoring of the opposite limb and infection is observed as some potential adverse events.
Orbital Sinus Blood Sampling
This technique requires high skill as a minor mistake can damage an animal’s eye. Only one blood sample is recommended to be withdrawn. A sample volume of 0.2 ml in case of recovery and up to 0.5 ml non-recovery experiment can be taken using a Pasteur pipette or capillary glass tube. One person is sufficient to perform blood sampling. Corneal ulceration, Retro-orbital Hemorrhage, keratitis, the rapture of globe pannus formation, micro-ophthalmic due to proptosis of the globe, damage to the optic nerve and other intraorbital structures, fracture of fragile bones of orbit and penetration of eye globe itself resulting in a loss of vitreous humor can be observed as untoward effects of Orbital sinus blood sampling (Solomon, Antunes, Chen, Bland, & Chien, 1985). So careful monitoring for adverse effects is necessary both before and after the performance. Check your animal for periorbital and post-operative lesions after thirty minutes of sampling. There should be a gap of at least two weeks between two bleedings.
Thoracic Blood Vessel Sampling
This technique is suitable for withdrawing large and single blood sample. This technique can be used when cardiac damage is needed to be avoided. Only one blood sample of up to 1ml should be taken using 25G needle, and one person is sufficient to take the sample. Sample can be taken from the aortic arch, abdominal vena cava or abdominal aorta. Withdraw the sample slowly so collapsing of vessels can be avoided. (Sorg et. al., 1964)
Cardiac Puncture Blood Sampling
Only one blood of up to 1 ml using 23 to 25G needle or 1 to 5ml syringe can be taken. One person is enough to withdraw blood via cardiac puncture. Take your sample slowly from the ventricle to avoid heart collapse. (Ungerstedt, 1991)
To withdraw Blood Sample from Rabbit
Requirements

Blood Collection from the Marginal Ear Vein
Considering sample volume, up to 8 samples can be withdrawn in a 24-hour period. A sample of up to 0.5 to 10ml can be taken from a rabbit, depending on the type of rabbit. A blood sample can be taken using a 19-23G butterfly needle. Two persons should be enough to perform the blood sampling; one person to restrain the animal and the other one to withdraw the blood sample. Infection, Hemorrhage or bruising can be observed as adverse events.
Blood Collection from the Lateral Saphenous Vein The simplest and most convenient site to collect
blood is from the lateral saphenous vein located at the leg. The attendant restrains the rabbit with the other person withdrawing blood. Clip the hair from a lateral saphenous vein on a lower rear limb. Occlude the vein, use alcohol and sterile wire gauze to clean and dry skin over the vein. Insert a 21G needle in an occluded vein by using a scalp vein set that is primed with sodium heparin, and collect the blood in a 3ml syringe that is already filled with 1ml air and is connected to the needle. Gently shake the syringe during blood collection so that blood clotting can be avoided. Apply gentle pressure at the puncture site for about 20 sec. (Williams & Kendall, 2015)
Blood Collection from Central Ear Artery
Rabbit’s ear should be shaved, and it should be extended away from the head to visualize the artery. Local anaesthesia should be applied 30 minutes prior to blood collection; wrapping your animal in a comfortable cloth is preferred to prevent accidental movement. Up to 200ml sample volume can be collected using a 20 to 22G needle. Maintain the rabbit’s ear in a lower position to aid blood collection. If no blood flows through your needle, try on the artery located at the proximal site to the previous one.
Blood Collection from Cardiac Puncture
Only one sample can be withdrawn as it is a terminal technique of blood collection; a total of 60 to 120ml blood volume can collect considering the rabbit size and heartbeat. 19 to 21G needle is used to take a sample from the ventricle slowly to prevent an animal’s heart from collapsing. Cardiac puncture is not recommended if there is a need to lavage peritoneum as blood can escape to the peritoneum cavity via this technique.
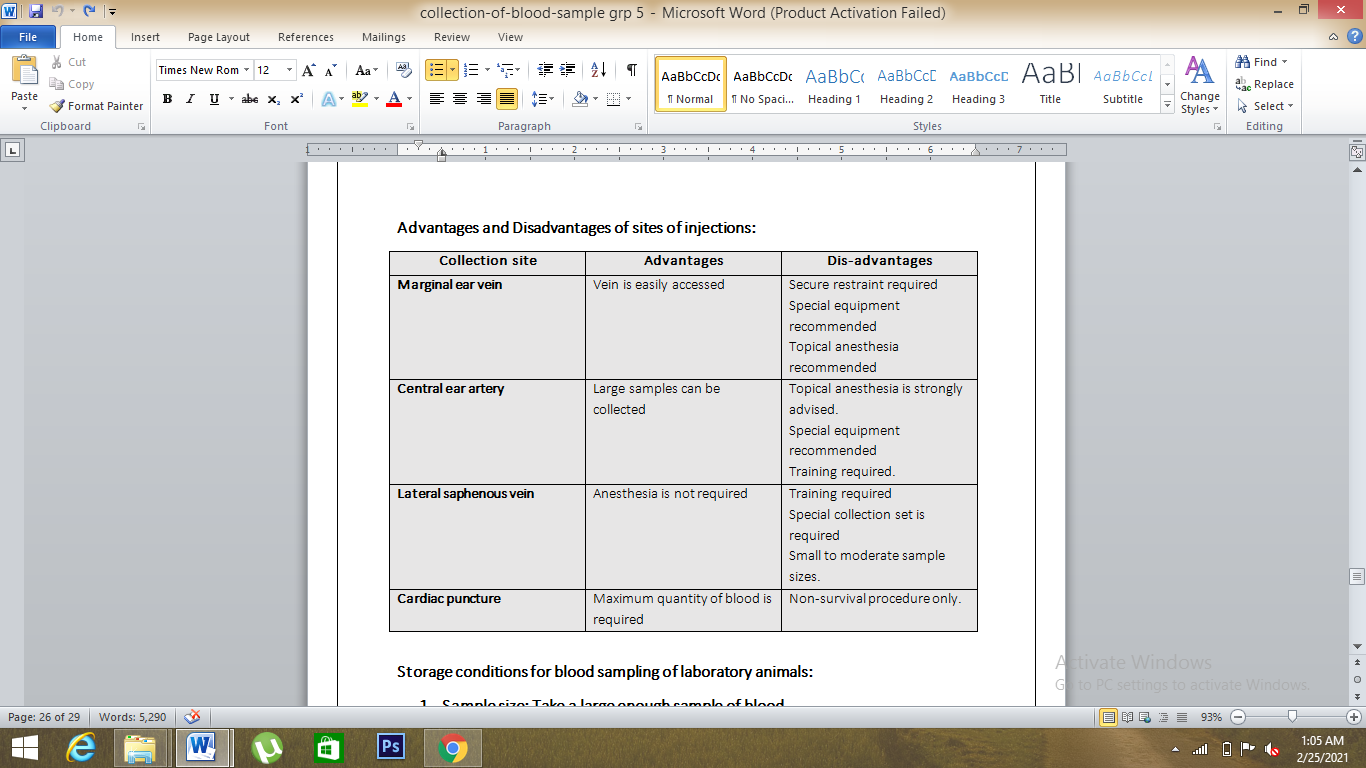
Comparison of different blood collection sites
Storage conditions for blood sampling of laboratory animals
Storage Time
Make sure to observe proper conditions during the storage of samples for safe and effective use of these samples in future.
Blood that is stored for more than three weeks loses its flexibility and is not able to fit in the smallest capillaries of the body.
The shelf life of blood is considered to be 3 weeks, and it has been shown in a study conducted at John Hopkins University that red blood cells lose their ability to transport oxygen.
Longer storage without refrigerating blood and poor temperatures at frozen conditions can risk the visibility of the blood sample.
Size of Sample
Take enough blood sample volume to run two either separate or duplicate of desired tests.
Test sample collected as soon as it is taken, usually within 4 hours of blood collection.
It is recommended to take 2 to 2.5X volume of blood that is required in an experiment or test.
Heparin, anti-coagulants, clot accelerators and other additives may be used
Temperature
For future use, blood samples should be stored or cultured in liquid nitrogen.
Typically three temperatures, including room temperature, refrigerated and frozen, are specified based on the sample under consideration.
Where room temperature is between 15 and 30-degree centigrade, the frozen temperature is 20-
Degree centigrade or below 20 and refrigeration temperature is referred to as between 2 and 10-degree centigrade.
Conclusion
Most pharmaceutical research relies on the usage of laboratory animals which manifests blood sample withdrawal from animals to perform the test under question. The article reviews preferred blood sample withdrawal techniques from common laboratory animals, which include rats, guinea pig, rabbit and mice. The article provides the researcher with the layout to make a decision for blood withdrawal technique and route, keeping in view the adverse effects of each route. Animal ethics should be the first consideration whenever planning any experiment; it should be observed to house experimental animals under the least stress for optimum results.
References
- Bardelmeijer, H., Buckle, T., Ouwehand, M., Beijnen, J., Schellens, J., & Van Tellingen, O. J. L. a. (2003). Cannulation of the jugular vein in mice: a method for serial withdrawal of blood samples. 37(3), 181-187.
- Birck, M. M., Tveden-Nyborg, P., Lindblad, M. M., & Lykkesfeldt, J. J. J. (2014). Non-terminal blood sampling techniques in guinea pigs. (92), e51982.
- Courtice, F. J. T. J. o. p. (1943). The blood volume of normal animals. 102(3), 290.
- Cuper, N. J., Verdaasdonk, R. M., De Roode, R., De Vooght, K. M., Viergever, M. A., Kalkman, C. J., & De Graaff, J. C. J. C. p. (2011). Visualizing veins with near-infrared light to facilitate blood withdrawal in children. 50(6), 508-512.
- Dang, V., Bao, S., Ault, A., Murray, C., McFarlane- Mills, J., Chiedi, C., . . . Rao, S. J. J. o. t. A. A. f. L. A. S. (2008). Efficacy and safety of five injectable anesthetic regimens for chronic blood collection from the anterior vena cava of guinea pigs. 47(6), 56-60.
- Kalisch, R., Elbel, G.-K., Gössl, C., Czisch, M., & Auer, D. P. J. N. (2001). Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 Tesla: implications for pharmacologic MRI. 14(4), 891- 898.
- Kowarski, C., Giancatarino, C., Kreamer, R., Brecht, D., & Kowarski, A. J. J. o. p. s. (1976). Measurement of sulfamethizole clearance rate by nonthrombogenic constant blood- withdrawal system. 65(3), 450-452.
- Matta, B. F., & Lam, A. M. J. T. J. o. t. A. S. o. A. (1997). The rate of blood withdrawal affects the accuracy of jugular venous bulb: oxygen saturation measurements. 86(4), 806-808.
- Parasuraman, S., Raveendran, R., Kesavan, R. J. J. o. p., & pharmacotherapeutics. (2010). Blood sample collection in small laboratory animals. 1(2), 87.
- Solomon, R. A., Antunes, J. L., Chen, R., Bland, L., & Chien, S. J. S. (1985). Decrease in cerebral blood flow in rats after experimental subarachnoid Hemorrhage: a new animal model. 16(1), 58-64.
- Sorg, D. A., Buckner, B. J. P. o. t. S. f. E. B., & Medicine. (1964). A simple method of obtaining venous blood from small laboratory animals. 115(4), 1131-1132.
- Ungerstedt, U. J. J. o. i. m. (1991). Microdialysis- principles and applications for studies in animals and man. 230(4), 365-373.
- Williams, W. R., & Kendall, L. V. J. L. a. (2015). Blood collection in the guinea pig (Cavia porcellus). 44(6), 207-208.
Cite this article
-
APA : Rashid, N., Ahmed, A., & Shahid, H. (2018). A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals. Global Immunological & Infectious Diseases Review, III(I), 1-7. https://doi.org/10.31703/giidr.2018(III-I).01
-
CHICAGO : Rashid, Nida, Ayesha Ahmed, and Hafsa Shahid. 2018. "A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals." Global Immunological & Infectious Diseases Review, III (I): 1-7 doi: 10.31703/giidr.2018(III-I).01
-
HARVARD : RASHID, N., AHMED, A. & SHAHID, H. 2018. A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals. Global Immunological & Infectious Diseases Review, III, 1-7.
-
MHRA : Rashid, Nida, Ayesha Ahmed, and Hafsa Shahid. 2018. "A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals." Global Immunological & Infectious Diseases Review, III: 1-7
-
MLA : Rashid, Nida, Ayesha Ahmed, and Hafsa Shahid. "A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals." Global Immunological & Infectious Diseases Review, III.I (2018): 1-7 Print.
-
OXFORD : Rashid, Nida, Ahmed, Ayesha, and Shahid, Hafsa (2018), "A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals", Global Immunological & Infectious Diseases Review, III (I), 1-7
-
TURABIAN : Rashid, Nida, Ayesha Ahmed, and Hafsa Shahid. "A Study on Different Conventional Techniques to Withdraw Blood from Experimental Animals." Global Immunological & Infectious Diseases Review III, no. I (2018): 1-7. https://doi.org/10.31703/giidr.2018(III-I).01
